[ad_1]
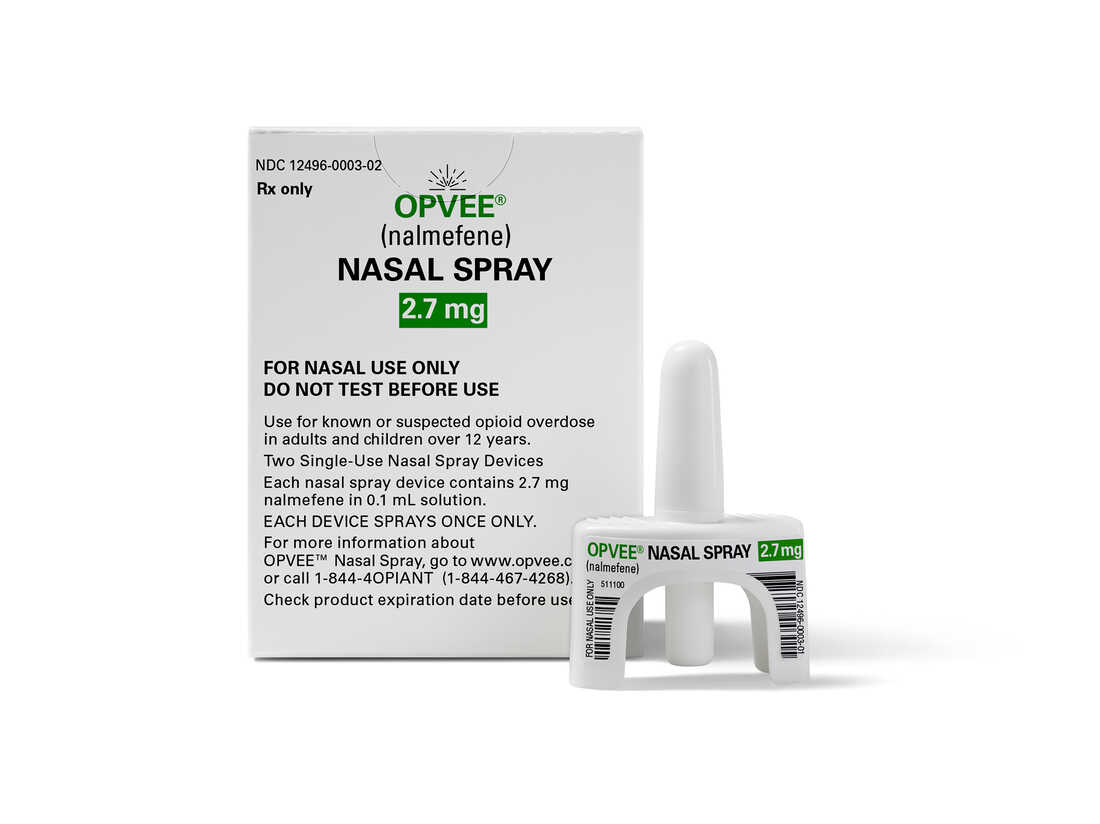
This image supplied by Indivior in Could 2023 demonstrates their drug Opvee. U.S. health regulators accepted the medicine to reverse overdoses caused by fentanyl and other strong opioids.
Indivior by way of AP
cover caption
toggle caption
Indivior by way of AP

This photo furnished by Indivior in May well 2023 exhibits their drug Opvee. U.S. wellness regulators authorized the medicine to reverse overdoses prompted by fentanyl and other effective opioids.
Indivior by means of AP
WASHINGTON — U.S. health regulators on Monday accepted a new simple-to-use model of a medicine to reverse overdoses prompted by fentanyl and other opioids driving the nation’s drug crisis.
Opvee is similar to naloxone, the daily life-conserving drug that has been utilized for decades to swiftly counter overdoses of heroin, fentanyl and prescription painkillers. Both equally perform by blocking the effects of opioids in the brain, which can restore regular respiratory and blood tension in people today who have recently overdosed.
The Food items and Drug Administration endorsed Opvee, a nasal spray update of the drug nalmefene, which was first authorized as an injection in the mid-1990s but later on eliminated from the market place owing to very low gross sales. Naloxone will come as both a nasal spray and injection.
It can be not promptly crystal clear how the new drug will be utilized in different ways compared to naloxone, and some specialists see prospective downsides to its extended-acting outcome. The drug will be readily available by means of prescription and is authorized for sufferers 12 and more mature.
In scientific tests funded by the federal authorities, Opvee accomplished very similar recovery effects to Narcan, the primary brand of naloxone nasal spray.
Opvee was developed by Opiant Prescribed drugs, which was a short while ago obtained by rival Indivior, maker of various medicines for opioid dependancy. Indivior expects to launch Opvee in Oct at the earliest.
As the opioid epidemic has shifted to fentanyl and other artificial opioids, researchers in the pharmaceutical market and the U.S. governing administration observed a new position for the drug.
Due to the fact fentanyl stays in the system extended than heroin and other opioids, some people today may possibly involve several doses of naloxone in excess of quite a few several hours to entirely reverse an overdose.
Scientists at the National Institutes of Health and fitness worked with pharmaceutical researchers on a nasal spray version of nalmefene that would promptly resuscitate people, even though also protecting them from relapse. Screening and development was funded by additional than $18 million in grants from the U.S. government’s Biomedical Innovative Analysis and Improvement Authority and the NIH, which also assisted design and style the reports.
“The total goal of this was to have a medicine that would last more time but also access into the mind quite promptly,” reported Dr. Nora Volkow, director of the Countrywide Institute on Drug Abuse.
Nonetheless, some gurus see potential downsides.
A facet influence of all opioid reversal medicines is that they develop intensive withdrawal signs like nausea, diarrhea, muscle cramps and nervousness. With naloxone, these signs might last 30 to 40 minutes.
Dr. Lewis Nelson of Rutgers University claims those issues can final 6 hrs or far more with nalmefene, necessitating additional therapy and management by health specialists.
“The hazard of very long-long lasting withdrawal is really actual and we consider to steer clear of it,” mentioned Nelson, an unexpected emergency medicine health practitioner and former adviser to the Food and drug administration on opioids.
Nelson claimed it can be quick plenty of to give a 2nd or 3rd dose of naloxone if it wears off.
“We’re not suffering from a naloxone lack where by we require to use an substitute,” he claimed. “We have plenty of it and it works properly well.”
The Food and drug administration acceptance comes as drug overdose fatalities inched up a little bit last yr following two significant leaps during the pandemic. Much more than 109,000 fatal overdoses have been recorded in 2022, in accordance to the most current figures from the Centers for Condition Regulate and Prevention.
A lot more than two-thirds of those people fatalities had been joined to fentanyl and other artificial opioids, which have mostly changed heroin and prescription opioids.
Naloxone has prolonged been at the center of governing administration efforts to fight the overdose crisis at the federal and nearby degrees. Police, firefighters and other initial responders routinely carry the drug. And officials in all 50 states have given orders to pharmacists to promote or dispense the drug without a prescription to any individual who needs it.
In the newest federal push, the Food and drug administration a short while ago permitted Narcan to be sold over the counter. The modify will allow the new variation of the drug to be stocked in grocery retailers, vending machines and other retail spots. The nasal spray — which incorporates current recommendations for regular users — is predicted to start this summer time. Emergent Biosolutions hasn’t yet declared a cost for the above-the-counter variation.
Indivior claimed it is however considering what to cost for its drug. It will compete in the exact sector as naloxone, wherever most buyers are local governments and local community groups that distribute to initially responders and people at danger of overdose. Indivior has instructed investors that Opvee could eventually create annual profits concerning $150 million to $250 million.
[ad_2]
Resource hyperlink




